Tempo-iAstro™
Human iPSC-derived Astrocytes
Tempo-iAstro™
Human iPSC-derived Astrocytes
Astrocytes, also known as Astroglia, belong to the family of glial cells in the human central and peripheral nervous system. They have been shown to have central roles in the maintenance of neuronal metabolism and neurotransmitter synthesis. They have been implicated in many human neurodegenerative disorders such as multiple sclerosis, amyotrophic lateral sclerosis (ALS), Alzheimer’s disorder, Huntington’s disease and Parkinson’s disease.
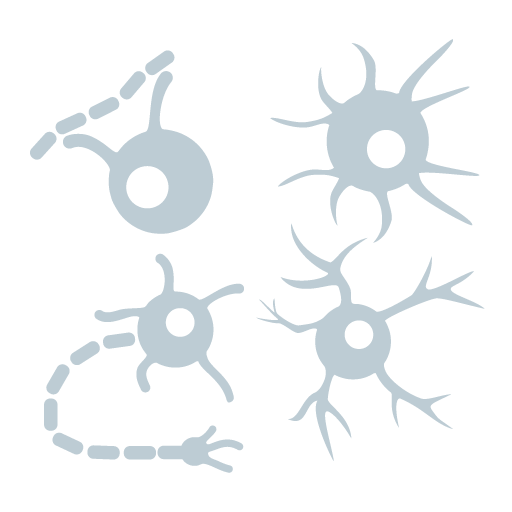
Tempo’s iAstro™: human iPSC-derived astrocytes are derived from integration-free induced pluripotent stem cell (iPSC) lines under a fully defined proprietary neural induction condition. Tempo’s iAstro cells are polarized structures when plated as a monolayer in culture and express astrocyte markers, GFAP, EAAT1, Aquaporin family genes, and S100beta, and they exhibit calcium wave activities (see figures shown above). There are two subtypes of human astrocytes: Type I and Type II. >90% of Tempo-iAstro™ astrocytes are Type II. If you need Type I astrocytes, please email Tempo Support.
Applications
Tempo-iAstro™ are intended for basic scientific research, drug discovery and therapeutics development use only. It is not a product for human testing or diagnostics.
Phenotypic Assays

High Content Imaging
Biomarker Discovery
Cytotoxicity Assays
Target Validation
Lead Optimization

Investigative Toxicology
Nonclinical Efficacy Evalutions

Live-cell Imaging
2D & 3D Cell Culture
Biomarker Authentication
Tempo’s “Build an organoid” is easy to scale!
Ready in DAYS NOT WEEKS, and simplifies assay and data analysis.

iOligo, iMG
ratio to 6:1, 3:1, 4:1
Specifications
>0.5×10^6 cells per 1ml of freezing medium (vial)
Long-term Storage: liquid nitrogen
Growth Properties: adherent
Storage: remove cryovials (dry ice packaging) and place the vial into liquid nitrogen for storage. Alternatively, thaw and use the cells immediately.
Technology used: an in-house developed proprietary non-viral, nucleic-acids-free, feeder-free, serum-free, and integration-free reprogramming technology.
QC: Sterility, Safety (BioSafety Level 2), HIV/viruses, bacteria, fungi: negative. Cell viability post-thawing (>90%)
Tempo-iAstro™ SKU101
Citations
- Enhanced Astrocyte Responses are Driven by a Genetic Risk Allele Associated with Multiple Sclerosis.
- The Genetic Multiple Sclerosis (MS) Risk Variant rs7665090-G Increases Astrocyte Responses and Lymphocyte Recruitment to White Matter Lesions.
- The Role of Astrocytes in Multiple Sclerosis. (Review)
- New Research to Uncover Cellular Mechanisms Driving Risk for Multiple Sclerosis. (Review)
- Cell Sources and Methods for Producing Organotypic in vitro Human Tissue Models
- Patient-Derived Orthotopic Xenografts and Cell Lines from Pediatric High-Grade Glioma Recapitulate the Heterogeneity of Histopathology, Molecular Signatures, and Drug Response.
- βA1-crystallin regulates glucose metabolism and mitochondrial function in mouse retinal astrocytes by modulating PTP1B activity.
- Patient-derived models recapitulate heterogeneity of molecular signatures and drug response in pediatric high-grade glioma
