Every middle school biology student is told the same lies.
Well, perhaps it’s more fair to say the same, “incredible oversimplifications of the truth”.
In biology 101, I was told that the nucleus is the “brain of the cell”, the membrane is the “wall of the cell” and the mitochondria is the “power plant of the cell”.
The power plant of the cell…
*cringe!*
Let’s talk about the many functions of mitochondria in the body, mitochondrial dysfunction and how in vitro bioassays help ensure the safety of drugs.
What do mitochondria do in the body?
The mitochondria make over 90% of the body’s energy in the form of adenosine triphosphate (ATP). ATP releases energy when it is broken down. This energy is vital to sustain life and support healthy organ function. The body’s main energy consumers are the brain, muscles, liver, kidneys, gastrointestinal tract, heart and lungs. As important as this role is, mitochondria are also key players in many other bodily processes (see the diagram below).
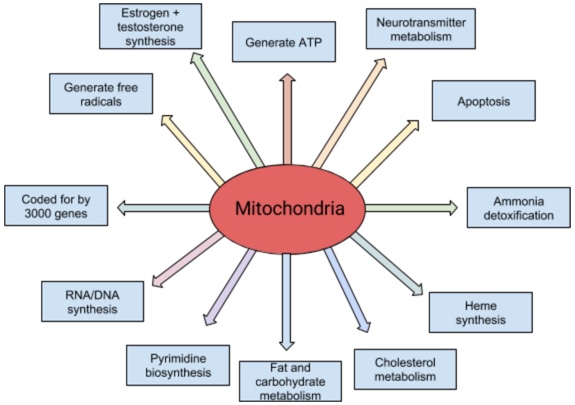
It takes about 3000 genes to make a mitochondrion (only about 3% of the genes necessary to make a mitochondrion are allocated to ATP production, by the way). More than 95% of these genes are involved in processes described above including most major cell metabolic pathways, production of the rate-limiting enzymes for pyrimidine biosynthesis (dihydroorotate dehydrogenase) and heme synthesis (d-amino levulinic acid synthetase) required to make hemoglobin and neurotransmitter metabolism.
As you can imagine from the above plethora of mitochondrial functions, mitochondrial dysfunction (see below) can lead to significant health issues affecting many of the body’s systems. At its most severe, it can even cause organ failure and death.
What is Mitochondrial Dysfunction?
Put simply mitochondrial dysfunction or mitochondrial dysfunction is the failure of mitochondria to function normally. When the mitochondria fail to meet the energy requirements of the body, symptoms of this insufficiency arise. These include: seizures, strokes, significant developmental delays, heart and kidney issues, problems with speech, sight, digestion, walking as well as many other symptoms. This can be difficult to diagnose as many of these symptoms overlap with other diseases. Thus mitochondrial disease is only usually suspected where three or more organ systems are affected.
While mitochondrial dysfunction is primarily known for its role in mitochondrial disease, it has been identified as an significant factor in many conditions and disorders including neurodegenerative disorders (like ALS, Parkinson’s, and Alzheimer’s), cardiomyopathies, metabolic syndrome, cancer, and obesity.
What causes mitochondrial dysfunction?
On a physiological level, mitochondrial dysfunction is caused by exposure to certain environmental factors (such as certain pharmaceutical drugs, occupational chemicals and cigarette smoke) or genetic abnormalities (of both mitochondrial and nuclear DNA).
Environmental factors include: certain pharmaceutical drugs, air pollution and particulates, certain occupational chemicals and other mutagens.
On a cellular level, mitochondrial dysfunction is caused by:
(1) a reduction in the electrical and chemical transmembrane potential of the inner mitochondrial membrane
(2) the improper functioning of the electron transport chain
or
(3) the impaired transport of critical metabolites into mitochondria
The above affects: the cell cycle, metabolism, cell viability, gene regulation and other key aspects of cellular growth and survival.
A common misconception is that since we inherit our mitochondrial DNA from our mother’s genetic mitochondrial disease is inherited from our mother’s only. However, genetic mutation includes both mitochondrial and nuclear DNA mutations. Mitochondrial diseases can affect any organ system, appear at any age, and, depending on where in our DNA the mutated gene is, be inherited from an autosome, the X chromosome, or maternally. Thus far, there is no cure and treatments only aim at treating symptoms.
Mitochondrial Dysfunction and Drugs
To clarify, by drugs is meant those prescribed by your doctor, not the illicit kind.
Why our mitochondria in particular may be sensitive to drugs is an interesting question. Sometimes referred to as the “canaries in a coalmine”, mitochondria seem to be particularly sensitive to certain drugs in particular. These include antimicrobials. The ancestry of mitochondria may play a role in this. Mitochondria have a bacterial ancestry, meaning that they first appeared in bacteria well before humans existed. This might explain why mitochondria are sensitive to antimicrobials: it’s in their genes (quite literally). For example certain antimicrobials work by block microbial ribosomes. It has been posited that drug toxicity may be in many cases be caused by damage to our cell’s mitochondria which results in a plethora of symptoms and disorders.
Developing safer drugs: Mitochondrial Assays
The use of specific and sensitive in vitro assays in early drug development helps researchers detect compounds which negatively affect mitochondrial function. This enables researchers to identify drug induced mitochondrial toxicity early in the development process and ensures drugs that reach the stage of clinical trials are safe. Such assays measure cytotoxicity and cellular metabolism to help with drug screening. As example is biosensors. Genetically-encoded biosensors can be incorporated into various cell lines including immortalized cancer cells, tumors, inducible pluripotent stem cells (iPSCs) and iPSC-derived cells. TempoMito™ is an example of a genetically-coded biosensor. It measures calcium fluctuations in the mitochondria to detect mitochondrial dysfunction. Mitochondrial dysfunction can also be measured indirectly by measuring cellular ATP productions using TempoATP™.
Is this the first time you’ve heard of mitochondrial dysfunction? Are you a researcher who uses assays like these? Let us know in the comments below or tweet us @TempoBioscience!
