In an article published in genengnews.com, the findings of a research study “Topological Morphogenesis of Neuroepithelial Organoids” discovered new information about cell-to-cell tissue connectivity that provides an organizational backbone for further organ development. Understanding cellular organizational development has important implications for transforming the process of developing organoids into a replicable process.
Article Background
Topology, the arrangement of connective tissue that determines cellular form and function, is a key element of cellular development. As tissues develop out of a few cells, simple topology evolves into topological morphogenesis. Morphogenetic processes, such as size, shape, and topological changes, occur as the cell develops. However, scientists have little idea what elements dictate these transitions and formations. Understanding organ topology is essential for proper characterization and engineering of tissues that will mimic human organs.
To explore morphogenetic processes, scientists in this study chose to investigate neuroepithelial organoids. Utilizing mouse embryonic stem cells, researchers found that the neuroepithelial organoids formed fluid-filled lumen, which are known to form the root of essential transport networks such as ventricles located in the brain. Furthermore, neuroepithelial organoids apically presented many passages which indicated that topological morphogenesis was present in the complex tissues.
Article Summary
Scientists from the Max Planck Institute of Molecular Cell Biology and Genetics investigated how embryonic development is directed and organized. To do so, they developed organoids using mouse embryonic stem cells that form a network of epithelia. Utilizing advanced microscopy, researchers were able to visualize dynamic changes deep within the organoids and see how environmental changes would influence development.
Their findings showed that the epithelium either self-fuses by fusing its two ends or joining with another separate epithelia. These patterns helped researchers discover a pharmacologically convenient pathway that regulates these fusion patterns utilizing different concentrations of HA130. Therefore, the shape and network connectivity of these organoids can be engineered to more accurately mimic human organs.
Importance to Organoid Trends
Organoid research and development is a rapidly growing field. In “What’s All the Fuss About Organoids?”, we discuss the various applications of how scientists use organoids, including studying embryonic development, modeling diseases, and evaluating drug toxicity.
In the figure below, we outline the potential utilities of 3D organoid research and development.
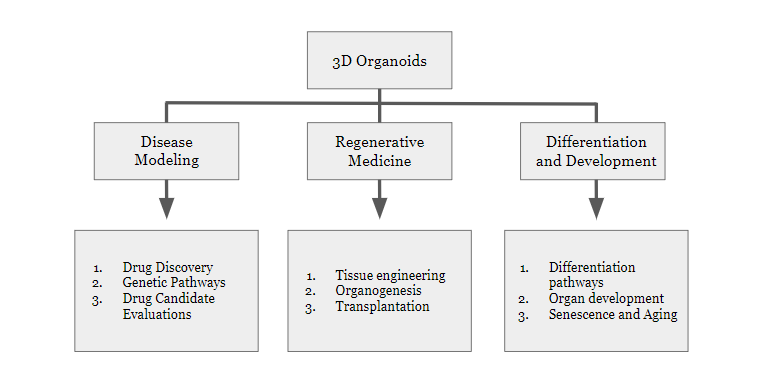
As it relates to the above article, the future of organoids relies on using accurate and relevant cell types to compose miniaturized multi-cell-type spheroid organoids. This system, also known as “body on a chip” highlights the new possibilities that the relationship between microfluidic technology and organoid cultures provides for scientific exploration. In a review article in springer.com, Tempo-iKidneyPod™, an organoid, was utilized in conjunction with microfluidic chips to examine how SARS-CoV-2 causes acute kidney injury (AKI).
Patient-derived organoids can circumvent the common issue of organ transplant rejections due to immune system reactivities. This introduces an exciting aspect of organoid modeling; biobanking of organoids allows fields such as disease epidemiology and developmental biology to have access to organ-specific tissues in bulk. Organoid biobanks have been useful in modeling cancer and cystic fibrosis, for example.
To create the most accurate organoid models, researchers are interested in understanding how organoids develop. As is discussed in this study, in knowing how connective tissue is structured, researchers are closer to understanding how to engineer patient-derived organoids.
Organoid Creation and Misconceptions
Organoids can be generated through a variety of sources; adult stem cell-containing tissue samples, single adult stem cells, or iPSC differentiation. Unlike spheroids, they need to be cultured in Matrigel or another extracellular matrix to support proper organoid development. When grown in media with specific growth factors, organoids can be grown indefinitely.
While organoids are promising as an accurate in vivo model, there are several misconceptions about organoids that should be considered. First, organoids are not exact replicas of organs and therefore calling organoids “mini-organs” is inaccurate. Additionally, organoids and spheroids are very different modeling methods and their differences should be highlighted and cannot be used interchangeably. Organoids are composed of multiple cell types and so ensuring proper proportionality of each cell type is essential to mimic the proportionality of cell types in real organs.
Relevance to Tempo Bioscience
Human iPSC-derived organoids are a key step to improved organ modeling since individual components and cell type cannot be easily obtained. Furthermore, as it pertains to this study, this source of cells also allows researchers to study the developmental process of organogenesis.
Products such as Tempo-iKidneyPod™, a 3D spheroid that is composed of human iPSC-derived kidney proximal tubules and podocyte cells, is a patient-specific multi-cell model that can be used to assess drug toxicity in the kidney. Additionally, Tempo offers multiple central nervous system relevant iPSC cell types (Tempo-iCort™, Tempo-iAstro™, Tempo-iOligo™, Tempo-iMG™/microglia) that can be combined to create a single-cell-type 3D spheroid or multi-cell-type 3D organoid by combining the cell types using specified ratios of neurons-to-glia. As previously mentioned, creating an accurate organoid model requires proper proportions of cell types cultured separately and then combined subsequently.
