Have you heard of the retinal pigment epithelium?
Well, the retinal pigment epithelium (or RPE) is located at the interface between the light-sensitive outer parts of the visual cells (photoreceptors) in the retina and the blood supply of the choroid in the eye (Figure 1). The choroid is a primarily vascular structure that lies between the retina and the sclera (white of the eye), and supplies blood to the outer part of the retina.
With its location, the RPE forms the outer blood-retinal barrier.
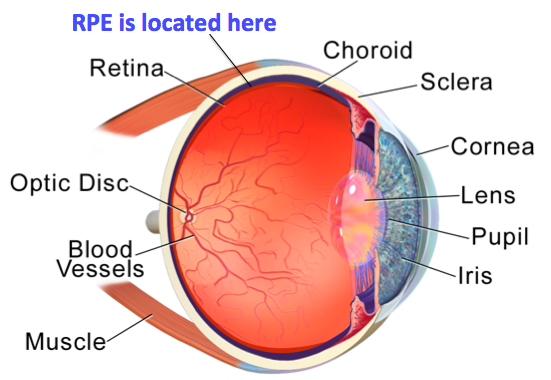
Figure 1: Anatomy of the human eye
What Does the RPE Look Like?
In vivo, the RPE comprises a monolayer of hexagonal cells cells that are connected to each other and tightly packed with pigment granules. This monolayer supports the photoreceptor cells e.g., rods and cones (Figure 2).
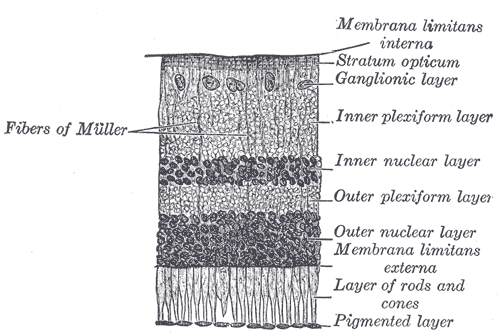
Figure 2: The retinal pigment epithelium (pigmented layer) in an illustrated section of a retina.
The Retinal Pigment Epithelium – a Jack of All Trades
The RPE’s most critical role is to provide support for retinal ganglion cells, and as part of a duo with the photoreceptor cells, the RPE makes an essential contribution to light detection in the eyes of all animal species.
In addition, the RPE itself boasts an impressive range of functions, which are outlined in the table just below.
| Light absorption | The RPE is packed with pigment granules and covers the inner surface of the eye globe (eyeball without its appendages), which makes it adept at absorbing scattered light, thus boosting the optical wiring of the eye. |
| Epithelial transport | From the subretinal space to the choroid:
The RPE transports electrolytes and water. From the blood to the photoreceptors: The RPE transports glucose and other nutrients such as retinol (Vitamin A), ascorbic acid, and fatty acids. Glucose transport is either basal or may be induced to meet changing metabolic demands, and this transport and its regulation is facilitated by the high expression of specific glucose transporters in the RPE. |
| Buffering of ions in the subretinal space | The RPE regulates and buffers the ions that occupy the subretinal space. This role is critical to the maintenance of photoreceptor excitability. |
| Visual cycle | Although the RPE is not a part of the neural retina, it is critical for function and survival of light sensitive photoreceptors. Of major importance is the regeneration of 11-cis retinal in the visual cycle. 11-cis retinal constitutes half of the rhodopsin molecule, a biological chemical that is critical for visual perception. |
| Phagocytosis | The membranes that line the outer segment of photosensitive cells (POS) are continuously exposed to photo-oxidative stress. To withstand this, POS go through a constant cycle of shedding their destroyed ends, and replacing them with new ends. The shed segments are phagocytosed and digested by RPE cells. |
| Secretion | To ensure communication with both the photoreceptors and the choroid blood cells that it interfaces with, the RPE secretes an impressive arsenal of factors and signaling molecules.
These include, but are not limited to: ATP, growth factors e.g., fibroblast growth factors, transforming growth factor-β, insulin-like growth factor-1, vascular endothelial growth factor, lens epithelium-derived growth factor, and interleukins. These signaling molecules play diverse and important roles in the maintaining structure in health eyes, as well as mediating the eye’s response to disease and physical injury. |
| Immune modulation | The inner eye is one of the few immune privileged sites in the body. These are sites that can tolerate the entry of antigens without the onset of an inflammatory cascade.
The RPE supports immune privilege both by forming a mechanical barrier that separates the inner eye from the bloodstream, and by mediating a direct immune modulating effects as necessary, to either prevent immune reactions in healthy eyes, or to activate immune responses in diseased eyes. |
What Color Are Your Eyes?
The melanosome is probably the most pronounced organelle in the cells of the RPE, and it’s main job is to synthesize and store the pigment melanin. Melanin actually refers to a group of naturally produced pigments whose members are also found in the skin and hair, and in humans, melanin is the primary determinant of skin color. Melanin also influences hair and eye color.
Melanosomes are located towards the apical surface of RPE cells throughout most of an individual’s lifetime, so that RPE melanin is primely positioned to absorb the light that traverses the photoreceptors, thus protecting against reflected light that might damage the visual image. In addition to its role in light absorption, melanin also protects against oxidative stress by scavenging reactive oxygen species and storing ferrous iron for oxidation and subsequent removal from RPE, the latter via the serum transport protein transferrin.
When It Goes Wrong – Diseases Associated with the RPE
Failures in any of the functions of the RPE can result in retinal degeneration, impairment or loss of visual function, or even blindness.
Let’s have a look (excuse the pun!) at some of the known RPE disorders:
| Disorder | About/Role of RPE |
| Albinism |
The melanosomes of the RPE cells fail to produce melanin. As well as the cosmetic effects of albinism, sufferers experience abnormal binocular vision. Albinism is inherited in an autosomal recessive manner, and exists in many forms, including oculocutaneous albinism that affects the skin, hair and eyes, and ocular albinism, which only affects the eyes. No treatment exists. |
|
Age-related macular degeneration (AMD) |
AMD is the leading cause of blindness in the U.S. The macula is a small spot that lies close to the retina. AMD is characterized by a sharp decline in central vision, and occurs when age-related macular damage impairs the ability to see objects that are straight ahead. Although the underlying cause for the macular damage is not fully understood yet, factors such as smoking, race and genetics play a role in its development. The appearance of pigmentation changes in the retina implicate the RPE in AMD, and current therapy efforts involve culturing sheets of RPE in vitro for implantation into the eyes. |
|
Retinitis pigmentosa (RM) |
A group of genetic disorders characterized by gradual but incomplete loss of vision. The underlying cause is the gradual depletion of rod photoreceptors in the back of the eye. Animal studies suggest that RM stems in part from a failure of the RPE to phagocytose the outer segments of aged rod cells, thus leading to a buildup of rod cell debris. The RPE itself is also destroyed during the course of RP. These disorders have been linked to at least 50 genes in humans, and while no cure exists, visual aids may improve life quality for sufferers. |
|
Diabetic retinopathy (DR) |
DR is a major complication in Type 1 diabetes, marked by abnormalities to the eye vasculature, vision loss and blindness. Degradation of the blood-retinal barrier and the retinal epithelium is well documented in DR, but the exact role of, or effects on, the RPE in this process is less well understood. |
RPE Cells in Research
Given the vast roles of the RPE, and the even greater number of diseases associated with its malfunction, RPE cells are a highly attractive research tool. Tempo-iRPETM are human iPS-derived retinal pigment epithelial cells that express biomarkers such as RPE-65, RLBP1, and BEST. These cells grow as a monolayer in culture and represent a versatile tool for studying age-related macular degeneration, retinitis pigmentosa, diabetic retinopathy, photoreceptor degeneration, gene expression, phagocytosis, and cellular interactions.
RPE cells in research have many applications such as:
- Controlled modeling of RPE-associated diseases
- Toxicity testing of new chemical entities aimed at treating RPE-associated diseases
- Phenotypic Screening (e.g., cytokine secretion, expression of differentiation markers) for new aspects to RPE function and disease
- In vitro assays using RPE cells
Do you work with RPE cells? What do you enjoy most (or least) about working with these cells? Get in touch by writing in the comments section. We’d love to hear from you!
Image Credits
- Figure 1. Blausen.com staff (2014). “Medical gallery of Blausen Medical 2014”
- Figure 2: Originally from: Henry Gray: Anatomy of the Human Body. PHILADELPHIA: LEA & FEBIGER, 1918
Article by Karen O’Hanlon Cohrt PhD. Contact Karen at karen@tempobioscience.com.
Karen O’Hanlon Cohrt is a Science Writer with a PhD in biotechnology from Maynooth University, Ireland (2011). After her PhD, Karen moved to Denmark and held postdoctoral positions in mycology and later in human cell cycle regulation, before moving to the world of drug discovery. Her broad research background provides the technical know-how to support scientists in diverse areas, and this in combination with her passion for writing helps her to keep abreast of exciting research developments as they unfold. Follow Karen on Twitter @KarenOHCohrt. Karen has been a science writer since 2014; you can find her other work on her portfolio.
