Welcome back to our Cell of the Month series! This time, we are talking about Kupffer cells – what they are, what they do, and why we should want to learn more about them!
Kupffer cells – the gatekeepers of the liver
First described by German anatomist Karl Wilhelm von Kupffer in 1876, Kupffer cells are a subset of macrophages that reside in the liver. Morphologically, they vary in size and have an irregular stellate shape, with bean-shaped nuclei. Elongated processes that extend from the cytoplasm help them to anchor to the luminal surface of sinusoidal endothelial cells.
Kupffer cells are the largest population of tissue-resident macrophages, constituting approx. 80-90 % of all macrophages found in the human body and 2-5 % of the total liver mass. As the liver’s primary immune sentinels, they form a crucial first line of defense against pathogens and foreign materials entering through the portal circulation (the system that carries blood from the abdominal organs to the liver).
In contrast to other tissue-resident macrophages, e.g., alveolar or splenic macrophages, Kupffer cells are directly exposed to the circulation which allows them to efficiently monitor and filter the blood supply. Their strategic location within the liver architecture enables them to perform their critical functions in maintaining liver homeostasis and immune surveillance.
A closer look at Kupffer cell functions
Kupffer cells play important biological roles locally (in the liver) and systemically. Most notably, they prevent pathogens and their toxic by-products, such as endotoxin, from entering the systemic circulation. As research into Kupffer cell biology evolves and new ways to study these cells emerge, we are gaining new insights into their roles in health and disease. Some of the best-characterised roles for Kupffer cells include:
Phagocytosis: Kupffer cells are professional phagocytes and the first cells to detect the presence of pathogen-associated molecular patterns and damage-associated molecular pathogens in the liver. They express various receptors on their surface, including several Toll-like receptors (TLR), C-type lectin receptors, complement receptors, Fc receptors and others. These receptors enable Kupffer cells to detect and engulf and destroy a wide range of matter, including bacterial and fungal pathogens and their toxins, tumour cells, cellular debris, apoptotic bodies, damaged or worn-out cells and foreign materials.
Antigen presentation: Kupffer cells are professional antigen-presenting cells which means in simple terms that they take up antigens, process them and present them to other immune cells. Kupffer cells express major histocompatibility complex class I (MHC-I), class II (MHC-II), which allows them to present antigens to CD8+ and CD4+ T cells. They also express several costimulatory molecules, such as CD80 and CD86 and CD40 which are necessary for T cell activation.
Cytokine and chemokine production: Upon activation, Kupffer cells release an arsenal of pro- or anti-inflammatory cytokines and chemokines depending on what triggered their activation. For example, studies in mice and in vitro assays based on immortalized cells have shown that, when exposed to bacterial LPS (which they detect via Toll-like receptor 4), Kupffer cells produce the pro-inflammatory cytokines TNF-α, IL-1, and IL-6, the chemokines CXCL10 and CCL2 to recruit neutrophils, reactive oxygen species to kill bacteria and activate lymphocytes, and eventually anti-inflammatory cytokines to halt inflammation when the threat is resolved (see 1 and references therein). The response to viral infection is different; TNF-α and other pro-inflammatory cytokines are produced to activate natural killer (NK) cells, while certain chemokines are produced to aid in the recruitment of T cells. The fine-tuned production of cytokines and chemokines allows Kupffer cells to carefully orchestrate immune responses, recruit the right immune cells for the threat/damage present, and regulate inflammation in the liver microenvironment.
Red blood cell clearance: One of the unique functions of Kupffer cells is their role in hemoglobin recycling through erythrocyte clearance. They efficiently remove senescent or damaged red blood cells from the circulation through phagocytosis, and this happens in response to several signals, including phenotypic changes, the presence of externalized phosphatidylserine and reduced expression of CD47 (the don’t eat me signal) the membranes of aged red blood cells. Following red blood cell phagocytosis, the hemoglobin chains are reused while the heme is processed to release iron and the bilirubin is eventually secreted back into the biliary system. This process is critical for maintaining iron homeostasis.
How do Kupffer cells compare to macrophages and microglia?
While Kupffer cells, macrophages and microglia are all phagocytes, they are not all the same. The table below summarizes their major differences with respect to tissue distribution, functions, longevity and biomarker expression.
| Kupffer cells | Microglia | Macrophages | |
| Tissue distribution | Liver-resident | Central nervous system (CNS)-resident | Widespread throughout the body except the skin which has its own phagocytic cell types |
| Key functions | Clearing pathogens and debris from portal blood
Key role in iron homeostasis as described above See previous section |
Immune surveillance in the CNS
Synaptic pruning during CNS development by removing weak or excess synaptic connections |
Diverse and location-dependant:
Phagocytosis of pathogens and cellular debris Antigen presentation to T cells and cytokine production for immune signaling Wound healing and tissue repair |
| Lifespan, renewal capacity | Intermediate life-span (months to years), and possess self-renewal capacity | Tend to be longer-lived than other phagocytes, can persist for years or for an entire life, can self-renew through local proliferation | Tend to have a shorter lifespan (days to weeks) than tissue-resident phagocytes, and are continuously replenished from monocytes |
| Biomarkers expressed (note this list is not exhaustive) | CD163, CD206, NR1H3, SpiC, CD68, S100AB | ApoE/variants, A1F1(= IBA1), hP2RY-12, TREM2, hGPR34, CXCR3, P2RX4, P2RX7, TRPM7, and CD11b/c | CD14, CD163, CD11b, CD16, CD68, CCR2, F4/80 |
Kupffer cells in disease – new therapeutic strategies?
Besides understanding their functions in the liver, Kupffer cells have been implicated in numerous diseases, and research to understand their role in disease pathobiology has paved the way for emerging therapeutic applications. Two notable examples include:
Non-alcoholic fatty liver disease (NAFLD)
According to the American Liver Foundation, non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver condition in the United States and is estimated to affect approx. 25 % of all adults. NAFLD is caused by a buildup of lipids in the liver (hepatic steatosis) and many individuals with the disease eventually develop a severe form of liver inflammation known as non-alcoholic steatohepatitis (NASH) which can be fatal. Along with monocyte-derived macrophages, Kupffer cells play a key role in the progression of NAFLD, NASH, and hepatocellular carcinoma. Once steatosis has begun, Kupffer cells secrete chemotactic substances such as chemokine C-C motif ligands (CCL) 1, 2, and 5. This facilitates the infiltration of monocytes which subsequently secrete vast amounts of pro-inflammatory cytokines. This activity further promotes hepatic steatosis leading to fibrosis progression and worsening of the disease. Efforts to target Kupffer cells as a therapeutic strategy for NAFLD include the use of naturally-ocurring and synthetic compounds to inhibit or reduce their pro-inflammatory activities and increase their anti-inflammatory activities, as well as chemokine (e.g. CCR2 and CCR5) antagonists to reduce Kupffer cell recruitment at the site of steatosis.
Viral hepatitis
Kupffer cells have been demonstrated to play a dual role in viral hepatitis caused by hepatitis B and C virus (HBV and HBC). Initially, they help to fight infection by detecting viruses through pattern recognition receptors, producing anti-viral interferons, and activating NK cells and T cells. However, during chronic viral infection, they can actually promote disease by maintaining a persistent inflammatory state that suppresses the effect of anti-viral T cell responses; this can occur via the production of immunosuppressive cytokines (IL-10, TGF-β) and expression of checkpoint molecules such as PD-L1.
An increased understanding in how Kupffer cells impact viral hepatitis may lead to new therapeutic avenues for these diseases. For example, the TLR8 agonist selgantolimod (SLGN) has previously been investigated in clinical trials for chronic HBV but until recently little was known regarding its action on immune effectors within the liver. A recent study by a team at INSERM in France aimed to characterize the transcriptomic and cytokine profiles of human primary Kupffer cells after exposure to SLGN. They found that the compound impairs HBV entry into hepatocytes, and that Kupffer cells exposed to the compound exhibited an upregulation in monocyte markers such as S100A12 and a downregulation in genes associated with Kupffer cell identity. They propose that SLGN can regulate Kupffer cell differentiation status and may be an important component of future combinations to cure HBV infection (1).
Beyond the examples above, Kupffer cells have been implicated in tumour progression in metastasis liver, cancer, liver disease in response to bacterial sepsis, and activation or rejection of liver following transplantation.
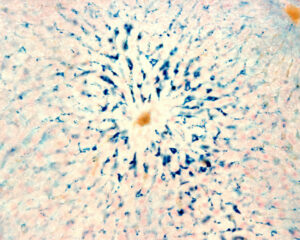
Kupffer cells are macrophages of the liver. Colloidal iron is a traceable stain for Kupffer cells’ phagocytosis activities in the body (blue).
Working with Kupffer cells?
Drug development efforts to target Kupffer cells continue to advance – from identification of genetic targets to cell signaling pathways. Choosing an appropriate cell-based experimental model is critical for validating drug development hypotheses and assay development efforts. While immortalized Kupffer cell lines offer practicality they are highly engineered and thus may not recapitulate the functions and phenotypes of their in vivo counterparts. Primary cells are a much more relevant physiological model, but they are tedious to cultivate and can be difficult to keep alive in cell culture. The emergence of induced pluripotent stem cell (iPSC)-derived Kupffer cells offers a promising middle ground; these cells are human, easy to culture and can be matured as long as the ‘appropriate’ cell culture conditions and media formulations are provided. As new insights emerge, we are optimistic that therapeutic approaches focusing on Kupffer cell function will grow in number.
If you are working with or considering working with Kupffer cells, and would like to learn more about the considerations mentioned above, or have any other question about working with this cell type, please do not hesitate to get in touch with us here. We are here to support your projects!
That was it for our introduction to Kupffer cells. Stay tuned for a future article about working with Kupffer cells in the lab!
References:
- Nguyen-Lefebvre AT, Horuzsko A. Kupffer Cell Metabolism and Function. J Enzymol Metab. 2015;1(1):101.
- Roca Suarez AA, Plissonnier ML, Grand X, et al. TLR8 agonist selgantolimod regulates Kupffer cell differentiation status and impairs HBV entry into hepatocytes via an IL-6-dependent mechanism. Gut. 2024 Nov 11;73(12):2012-2022.
Karen O’Hanlon Cohrt is an independent Science Writer with a PhD in biotechnology from Maynooth University, Ireland (2011). After her PhD, Karen relocated to Denmark where she held postdoctoral positions in mycology and later in human cell cycle regulation, before moving to the world of drug discovery. Karen has been a full-time science writer since 2017, and has since then held numerous contract roles in science communication and editing spanning diverse topics including diagnostics, molecular biology, and gene therapy. Her broad research background provides the technical know-how to support scientists in diverse areas, and this in combination with her passion for learning helps her to keep abreast of exciting research developments as they unfold. Karen is currently based in Ireland, and you can follow her on Linkedin here.
